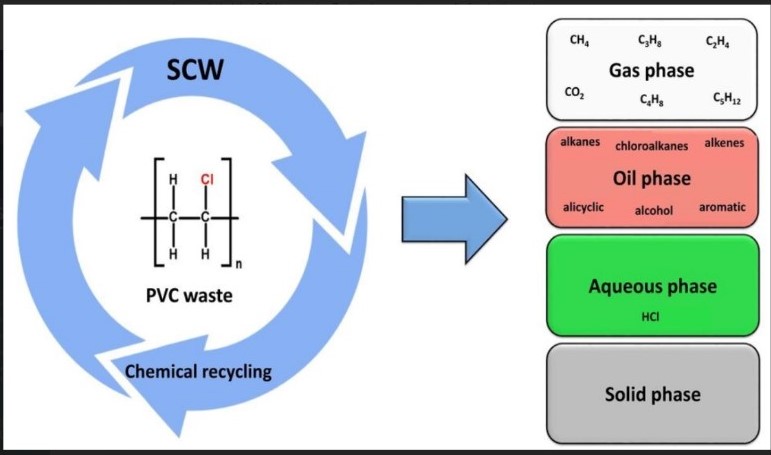
The chemical degradation of PVC waste in SCW between 400 and 425 ◦C and reaction times from 30 to 60 min was studied. The PVC waste in SCW decomposed into the gas, oil, water soluble, and solid phases. The highest yield of the gas and oil phases was achieved at the temperature of 425 ◦C after 60 min. By increasing the reaction time at 400 ◦C, the yield of chloride ions in the aqueous phase increased and reached the maximum at 60 min. The gas and oil phases contained many valuable compounds similar to crude oil. Alkanes and chloroalkanes; alkenes, alicyclic, and aromatic hydrocarbons; as well as alcohols were the main groups of hydrocarbons in the oil phase, while the gas phase contained only light hydrocarbons (C1–C6 ), CO2, and small amounts of H2 . This confirmed that the largest chlorine content remains in the aqueous phase and does not pass into the gas phase. It can be concluded that SCW presents effective decomposition media for plastic waste.